what is the electron configuration of fe|electron configuration of all elements : Tagatay Iron (Fe, Fe 2+, Fe 3+) Read my article in Science Education based on my .
An instructional video on how to reset your Kryptonite Combination Cable (KryptoFlex series include: 1018, 1218, 1230, 1565). Register your combination here!.
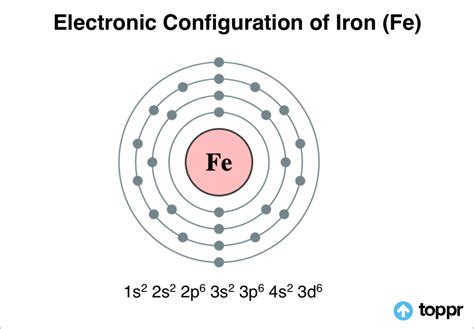
what is the electron configuration of fe,In order to write the Iron electron configuration we first need to know the number of electrons for the Fe atom (there are 26 electrons). Once we have the configuration for Fe, the ions are simple. When we write the configuration we'll put all 26 electrons in .In order to write the Calcium electron configuration we first need to know the .
Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s .
How to Write the Electron Configuration for Nitrogen (N) Nitrogen is the seventh .
what is the electron configuration of fe electron configuration of all elementsIn order to write the Mg electron configuration we first need to know the .Iron (Fe, Fe 2+, Fe 3+) Read my article in Science Education based on my .Iron (Fe, Fe 2+, Fe 3+) Read my article in Science Education based on my .In order to write the Argon electron configuration we first need to know the .How to Write the Electron Configuration for Oxygen. Oxygen is the eighth element .Electronic Configuration of Iron. The chemical element iron has the atomic number 26 and the symbol Fe (from Latin: Ferrum). It’s a .
Mar 23, 2023 Electronic Configuration of Iron. Iron, a transition element in Periodic Group VIII, is the fourth most abundant element in the Earth’s crust in terms of mass, and the second . The atomic number of Fe is 26, which means that its atoms contain 26 protons in their nuclei, and if neutral, 26 electrons in their electron clouds. The ground state electron configuration of Fe is: . 61K views 4 years ago. In order to write the electron configuration for Iron (Fe) we first need to know the number of electrons for the Fe atom (there are 26 electrons). When we write the.
Electron Configuration for Fe. The iron electron configuration is one of the other significant areas of study for scholars of chemistry. The electron . The Electronic Configuration of Iron. A chemical element with an atomic number 26 and a symbol Fe represents it, Iron is a very common element that is found. .What is the electron configuration and orbital diagram of: Na + P 3– Al 2+ Fe 2+ Sm 3+ Solution. First, write out the electron configuration for each parent atom. We have chosen to show the full, unabbreviated .

The iron’s electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Valence electrons are a single outer shell electron in an atom’s outer shell that is responsible for the atom’s chemical characteristics. In other words, a valence electron is an atom’s electron that may be transferred to or shared with another atom and is placed . In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x. The energy level, "n", can be determined based on the periodic table, simply by looking at the row number in which the element is in. However, there is an exception for the d-block and f-block, in which the energy level, "n" for the d block is .

Start with the electron configuration of the neutral atom. It is sufficient to use the noble gas configuration. Fe: [Ar] 4s 2 3d 6. Then, remove (or add) electrons to reflect the oxidation state of the metal. Remember to remove electrons from the orbital with the highest principal quantum number first! Fe(III) has lost 3 electrons relative to . An atom's electron configuration describes the way its electrons fill sublevels when the atom is in its ground state. Atoms seek the most stable electron configuration, so sublevels are half-filled or fully-filled .
electron configuration of all elements2.4 Electron Configurations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to .. For Fe, Z=26; there are thus 23 electrons to distribute for Fe^(3+). 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)3d^(5) Chemistry . Science . What is the electron configuration for #Fe^(3+)#? Chemistry Electron Configuration Electron Configuration. 1 Answer anor277 Jun 21, 2016
Atomic number of F e 2 + (Iron) = 26. Number of electron = Atomic number - charge. View the full answer. Answer.
The Fe2+ ion has two fewer electrons than a neutral Fe atom, for a total of 24 electrons. Therefore, its electron configuration will have 24 electrons. 1s22s22p63s23p63d6. As you can see, the two electrons that were transferred from the Fe atom to form the Fe2+ ion came from the 4s sublevel. .
Electron Configurations. The content that follows is the substance of General Chemistry Lecture 26. In this lecture we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the relationship of electron configuration to the periodic properties of the elements. . Fe 3+ 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5. One .
Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right. . 56 Fe Electron configuration [Ar] 3d 6 4s 2 CAS number: 7439-89-6 ChemSpider ID:What is the electron configuration for the Fe 3+ ion? Please explain why . a.) [Ne]3s 2 3p 10. b.) [Ar]4s 1 3d 6. c.)[Ar]4s 0 3d 7. d.) [Ar]4s 2 3d 9. e.)[Ar]4s 0 3d 5 The electron configuration for the iron (III) ion is: 1s2s22p63s23p63d5. The element iron, Fe, has the atomic number 26, which is the number of protons in its atomic nuclei. A neutral iron atom has 26 protons and 26 electrons. In order to form a 3+ ion, it must lose three electrons.what is the correct electronic configration of the central atom in K4[Fe(CN)6] based on crystal field theory?
what is the electron configuration of feElectron configurations of the elements (data page) This page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. For phosphorus (element 15) as an example, the .
Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Electron configuration of Iron (Fe) [Ar] 3d 6 4s 2: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2: 2, 8, 14, 2: 27: Electron configuration of Cobalt (Co) [Ar] 3d 7 4s 2: 1s 2 2s 2 2p 6 3s 2 3p . Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic structure.The chemical symbol for Iron is Fe. Electron Configuration and Oxidation States of Iron. Electron configuration of Iron is [Ar] 3d6 4s2. Possible oxidation states are +2,3. Electron Configuration. The periodic . The number of electrons in the valence shell of iron (Fe) can be used to calculate the electronic configuration of the various oxidation states. Fe2+ F e 2 +: Iron now has an electron configuration of [Ar]3d6 [ A r] 3 d 6 after losing two electrons from its 4s orbital. The iron ion acquires a 2+ charge as a result.
The electronic configuration of the Iron in its ground state is 1s2 2s2 2p6 3s2 3p6 3d6 4s2 and is also written as [Ar] 3d6 4s2. When an Iron atom donates two of its electrons, it attains a charge of +2. Any element that donates electrons gets a positive charge, whereas the elements that accept electrons gain a negative charge.
what is the electron configuration of fe|electron configuration of all elements
PH0 · zr4+ electron configuration
PH1 · molecular orbital configuration of f2
PH2 · full electron configuration calculator
PH3 · explain electron configuration
PH4 · electron configuration of all elements
PH5 · electron configuration chart
PH6 · condensed electron configuration fe
PH7 · abbreviated electron configuration for cs
PH8 · Iba pa